Scientists Say: Osmosis
This word describes how liquids can move across membranes to equalize the amount of a substance dissolved in them
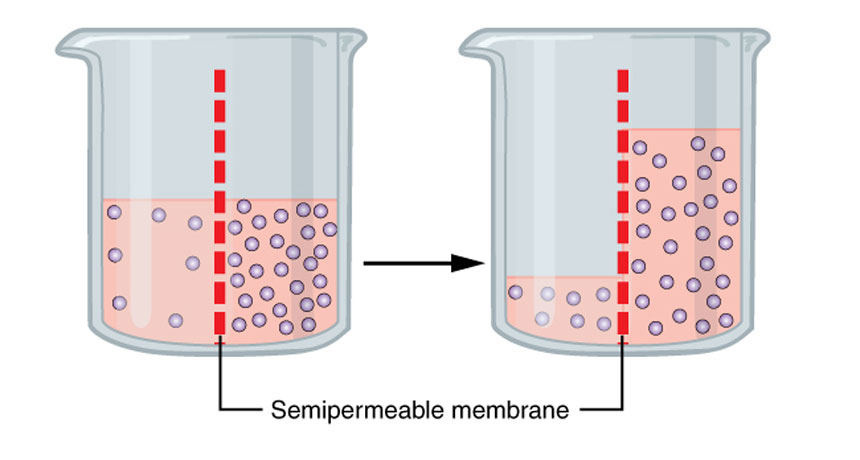
On the left, there are few molecules per volume of solution; on the right are many more. A somewhat porous membrane separates them. If microscopic holes in the membrane are big enough (but not too big), the liquid will move from left to right — through that membrane. And it will continue to do that until the concentration of the particles per unit volume is equal in the solution on each side of the membrane.
OpenStax College/Wikimedia Commons (CC BY 3.0)